Abstract
Introduction: Salvage immunochemotherapy (IC), followed by high-dose chemotherapy with autologous stem cell transplantation if chemosensitive is standard-of-care second-line (2L) therapy (tx) for fit patients (pts) with relapsed/refractory (R/R) diffuse large B cell lymphoma (DLBCL) when treated with first-line (1L) R-CHOP as shown in the CORAL study. Outcomes following receipt of salvage IC in the 2L setting for pts with DLBCL or high grade B cell lymphoma/B cell lymphoma unclassifiable (HGBL/BCLU) receiving intensive 1L tx remain unknown, and may be worse than those reported in CORAL given prior exposure to higher-dose IC in 1L setting. Here we report the results of a multicenter retrospective analysis of R/R DLBCL and HGBL/BCLU pts treated with intensive 1L tx who receive standard salvage 2L tx.
Methods: Inclusion criteria were histologic diagnosis of DLBCL or HGBL/BCLU, R/R disease following 1L tx with R-EPOCH, R-HyperCVAD or R-CODOX-M/IVAC and receipt of 2L tx with R-ICE, R-DHAP, R-DHAC, R-ESHAP or R-GDP. Exclusion criteria were HIV positivity, post-transplant lymphoproliferative disorder, prior chronic lymphocytic leukemia and inadequate data. Therapy was given at the discretion of the treating physician. Progression free survival (PFS) was defined as the interval between time of first relapse or primary refractory disease and disease progression, change in therapy if no disease response or last follow-up in remission, and overall survival (OS) between time of first relapse or primary refractory disease and death or last follow-up while alive. Pts were treated from 2007-2017 and data were censored on 10/15/17.
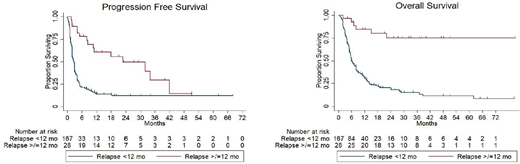
Results: A total of 195 pts treated at 20 US and Canadian academic medical centers were included. Clinicopathologic characteristics at time of R/R disease were 39% age >60 years, 62% male, 77% stage III-IV, 72% elevated LDH, 24% bone marrow (BM) involvement, 28% B symptoms present, 44% extranodal (EN) disease at >1 site, 19% ECOG performance status (PS) >1, 49% with International Prognostic Index score (IPI) ≥3, 46% HGBL/BCLU histology, 49% Ki67 ≥90%, and 61% germinal center (GCB) cell of origin (COO) by Hans algorithm. Of pts with available fluorescence in situ hybridization (FISH) data, 51%, 45% and 30% demonstrated MYC, BCL2 and BCL6 rearrangements (-R), respectively, and 37% were double hit lymphoma (DHL). Tx received in 1L were R-EPOCH in 82%, R-HyperCVAD in 16% and R-CODOX-M/IVAC in 2% of pts. R-ICE was received by 64% and other platinum-containing regimens by 36% as 2L tx. Most (86%) pts relapsed within 12 months (mo) of completion of 1L tx (early) and 58% of pts had primary refractory disease. For all pts, the median length of follow-up was 25.0 mo with a median PFS of 3.0 mo and median OS of 8.0 mo. Overall response rate to 2L tx among all pts was 44% (23% complete response [CR] and 21% partial response [PR]), and 48% with progressive disease. Pts achieving CR had significantly longer median PFS (32.0 vs 4.0 mo, p = 0.0001) and OS (not reached vs 13.0 mo, p = 0.0004) as compared to pts achieving PR. In pts who achieved CR or PR following 2L tx, 64% received consolidative transplant (42 autologous and 13 allogeneic) and achieved a median PFS and OS of 18.3 mo and 62.0 mo, respectively. As compared to pts relapsing ≥12 mo after completion of 1L tx (late), pts relapsing early were less likely to achieve CR (17% vs. 61%, p=0.0001) and experienced significantly shorter median PFS (2.8 vs. 23.0 mo, p<0.0001) and median OS (6.0 mo vs. not yet reached, p<0.0001). Univariate analysis incorporating clinicopathologic characteristics at the time of relapse demonstrated elevated LDH, stage III-IV disease, IPI ≥3, BM involvement, GCB COO, HGBL/BCLU histology, MYC-R, BCL2-R, DHL and early relapse to have a statistically significant increased hazard ratio (HR) for progression. All of these factors, as well as EN disease at >1 site, B symptoms, ECOG PS >1 and Ki67 ≥90%, but not BCL2-R, demonstrated a statistically significant increased HR for death. Multivariate analysis demonstrated only early relapse to have a statistically significant increased HR for progression (HR 2.47, p=0.024) and death (HR 5.90, p=0.001).
Conclusion: Relapse <12 mo from completion of intensive 1L tx is associated with extremely poor outcomes in pts with DLBCL and HGBL/BCLU treated with standard salvage 2L tx. Novel therapeutics, including chimeric antigen receptor-modified T cell (CART) tx, should be investigated as 2L tx in this pt population.
Maddocks:Pharmacyclics: Research Funding; BMS: Research Funding; Teva: Honoraria; AstraZeneca: Honoraria; Novartis: Research Funding; Pharmacyclics/Janssen: Honoraria; Merck: Research Funding. Wagner-Johnston:Novartis: Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; ASTEX: Research Funding; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Merck: Research Funding; JUNO: Honoraria, Membership on an entity's Board of Directors or advisory committees. Karmali:Gilead: Speakers Bureau; AstraZeneca: Speakers Bureau. Kahl:Genentech: Consultancy; Juno: Consultancy; Celgene: Consultancy; Seattle Genetics: Consultancy; ADC Therapeutics: Consultancy; Gilead: Consultancy; Acerta: Consultancy; CTI: Consultancy; Abbvie: Consultancy; AstraZeneca: Consultancy. Cohen:AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Research Funding; Infinity Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding; Millennium: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees; BioInvent: Consultancy; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Research Funding; Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Reddy:MEI Pharma: Research Funding. Ramchandren:Seattle Genetics: Consultancy, Research Funding; Pharmacyclics LLC an AbbVie Company: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy; Merck: Research Funding; Janssen: Consultancy, Research Funding. Diefenbach:Merck: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Incyte: Research Funding; Trillium: Research Funding; Millenium/Takeda: Research Funding; Denovo: Research Funding; Acerta: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Genentech: Consultancy. Olszewski:TG Therapeutics: Research Funding; Genentech: Research Funding; Spectrum Pharmaceuticals: Consultancy, Research Funding. Barta:Janssen: Membership on an entity's Board of Directors or advisory committees; Merck, Takeda, Celgene, Seattle Genetics, Bayer: Research Funding. Hill:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Genentech: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding; Seattle Genetics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees. Assouline:Roche: Honoraria, Research Funding, Speakers Bureau; Novartis: Research Funding; BMS: Honoraria, Research Funding, Speakers Bureau; Pfizer: Honoraria, Research Funding, Speakers Bureau; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Landsburg:Curis: Consultancy, Research Funding; Takeda: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract